Introduction
High-quality science education cultivates scientific understanding, sharpens critical thinking, and develops problem-solving skills – building a strong science-based society (Ploj Virtič, 2022). Science teaching strategies motivate students to interact during discussion, especially in chemistry, where lessons are complex and challenging to learn (Reid et al., 2022; Schmidt et al., 2018). Active learning strategies, such as problem-based learning, task-based learning, and hands-on experiments, have improved student comprehension and motivation (Borja & Mutya, 2024; Erickson et al., 2020; Syahfutra & Niah, 2019). Nevertheless, the conventional style of teaching has been continuously employed by many academic institutions, which may not sufficiently bridge learning gaps in understanding abstract scientific concepts. To address this problem, creative and innovative educational approaches must be utilized to align with global demands while promoting economic growth (Auld & Morris, 2019).
Despite decades of educational reforms, Filipino students continue to struggle in science, as shown by consistently low performances in international assessments. In 2018 and 2022 , Filipino students' achievement in science consistently ranked below the OECD average in Program for International Student Assessment (PISA) cycles, placing them among the lowest among participating countries (Bernardo et al., 2023; Lapinid et al., 2022). Similar trends were observed in the Trends in International Mathematics and Science Study (TIMSS), which highlighted learning gaps on a large scale, particularly in students' understanding of key scientific concepts(Ornedo, 2022). Such consistently low performance reflects the urgent need to enhance the instruction of science in the Philippines. A significant cause of poor performance isthe absence of interesting lessons and motivating materials that would help students conceptualize theirrules(Cayabas&Sumeg-ang, 2023; Co et al., 2021).To mitigate this challenge, the creation of inventive instructional resources helps promote active participation and develop a more comprehensive knowledge of complex science concepts, especially in chemistry.
However, one of the reasons behind the learning difficulties in chemistry is the students’ struggle to make connections between the macroscopic, submicroscopic, and symbolic levels of representations, often called the “triplet relationship” in chemistry (Kiernan et al., 2024). The difficulty of these representations is noticeable in content areas such as molecular structure and bonding, where typical chalk talk explanations do not provide the spatial and conceptual support that can be taken up by students (Moya, 2014). These limitations are even more evident when teaching complex topics such as IUPAC nomenclature and the three-dimensional structure of organic compounds, where students have to mentally form a three-dimensional configuration of carbon chains and the position of functional groups (Arteaga et al., 2025). To lessen the problem, an improvised molecular model, either virtual or physical, has been suggested for its effectiveness in enhancing visualization, spatial reasoning, and understanding (Febliza et al., 2025; Muilwijk & Lazonder, 2023). This tactile and visual approach allows students to physically build and pick up molecules, making abstract nomenclature concrete for students to better understand (Vayakone, 2024).
The educational theoretical framework for employing hands-on tools such as molecular models is based on constructivist learning theory, arguing that students learn better when they are actively involved and have the opportunity for personal experience in constructing understanding (Devi, 2019). Through kinesthetic learning, students interact with a three-dimensional representation of abstract constructs (Bansil & Yabut, 2025). This serves as a cognitive linkage between these layers, allowing students to easily visualize structures and bond formations, and spatial relationships. In this regard, improvised or low-cost molecular model kits offer a strategic teaching alternative that aligns with constructivism and is well-suited to the scarcity of learning resources in Philippine classrooms.
Several efforts illustrate the success of this approach. For instance, a meta-analysis conducted by Muilwijk and Lazonder (2023) found that students who were taught using physical and virtual models experienced significantly greater academic improvement compared to those taught using well-established instructional approaches. Locally,Moya (2014) found that active learning strategies significantly improved student learning for Chemistry 101 at Samar State University. Similarly,specialized recycled-material kits were found to increase student participation and achievement at public senior high schools (Dipalac & Castillo, 2024; Sagcal et al., 2017). These materials are highly valuable, as many schools in the Philippines lack laboratory equipment (Caballes et al., 2024; Hinampas & Fajardo, 2024).
Based on these contextual and instructional challenges, this study evaluated the effectiveness of an improvised molecular kit in enhancing students’ academic performance and perception of organic chemistry. In particular, it investigated whether active engagement in DIY molecular models can enhance students' understanding of chemical bonding and the reaction mechanisms of functional groups. Through this innovative teaching aid, the study supports the development of affordable, practical, and effective strategies for enhancing science education in resource-constrained environments. Lastly, this offered significant perspectives on how interactive and visual learning methods can make abstract chemistry concepts more concrete to enhance students’ understanding.
Methodology
Research Design
This research employed a pretest-posttest quasi-experimental design to evaluate the efficacy of an improvised molecular kit on students’ academic performance in organic chemistry, involving both control and experimental groups. This design enabled a systematic comparison of the learning outcomes between students who used the molecular kits and those who followed conventional instruction. As Kuś (2024) noted, this design is crucial for establishing the causal relationships between variables, particularly in education-related studies where random assignment is often not feasible. Through the use of this approach, the study generated empirical evidence of the efficacy of experiential learning tools in widening students’ knowledge and understanding of organic chemistry.
Participants
There were 90 participants involved in this study, selected through a purposive sampling method. They were divided into two groups: the control group, comprising 45 students, and the experimental group, consisting of 45 students from a public school in Cebu, Philippines. The groups were formed based on their science grades during the first quarter to ensure comparable levels of academic performance between the control and experimental groups. This sampling technique enables researchers to create groups with similar traits, reducing variability and enhancing the reliability and validity of comparative analyses (Campbell et al., 2020). In terms of demographic profile in terms of gender, in the control group, 24 out of 45 students (53.33%) were female and 21 (46.67%) were male, while in the experimental group, 26 out of 45 students (57.78%) were female and 19 (42.22%) were male, indicating a relatively balanced gender distribution in both groups.
Instruments
To evaluate students’ performance and their perception of the improvised molecular kit, several instruments were used in the study. The first instrument used for the pretest and post-test was composed of 120 items tailored to assess students’ performance in naming and writing the structures of functional groups, specifically alcohol, aldehyde, carboxylic acid, ester, ether, and ketone. This instrument was subjected to validity testing using Lawshe’s Content Validity Ratio (CVR) in terms of the three indicators: essential, helpful but not essential, and not essential. Experts with diverse academic backgrounds were selected to conduct the validation. They were specialists in chemistry education and research instrumentation with over five years of teaching experience. Expert reviews are necessary to further support the validity of the content, enhancing the clarity and precision of the test items (Rubio et al., 2003).
Analysis of the CVI results showed that items related to alcohol, carboxylic acid, and ester received a maximum score of (CVI = 1), signifying a high degree of alignment with the learning objectives. However, with a CVI between 0.9 and 0.933, aldehyde, ketone, and ether functional groups require minor revisions to enhance clarity, accuracy, and alignment with standard nomenclature rules. The reliability test using Cronbach’s Alpha produced a strong coefficient of 0.912,indicating the assessment instrument’s strong internal consistency. The knowledge test's internal consistency was further supported by KR-20 = 0.894 and KR-21 = 0.881, indicating strong reliability. The discrimination power of the items ranged from 0.36 to 0.81, and the difficulty coefficients were between 0.42 and 0.78, indicating that the test items were of moderate difficulty and effectively differentiated between high- and low-performing students.
To supplement the students’ assessment, a survey instrument was disseminated to assess students' attitudes toward using an improvised molecular kit, employing a four-point Likert scale that ranged from "strongly disagree" to "strongly agree."Lawshe’s (1975) CVR method was again utilized to validate the instrument, ensuring that each item was critically assessed for relevance and necessity by a panel of research instrumentation experts. Exploratory Factor Analysis (EFA) was also performed, resulting in the retention of 10 out of 30 original items, each demonstrating high factor loadings between 0.695 and 0.852, which reflects a clearly defined construct. It revealed a unidimensional structure, with only one factor extracted based on eigenvalue criteria, explaining a substantial portion of the variance. The Kaiser-Meyer-Olkin (KMO) yielded a value of 0.832, which means that the sample was suitable for factor analysis. At the same time, Bartlett's test of sphericity (χ² = 315.582, df= 45,p< .001) confirmed significant intercorrelations among items. The final instrument demonstrated excellent reliability, confirming its internal consistency with a Cronbach’s alpha .926.
Data-gathering Procedure
Prior to data gathering, the researcher sought approval from the school principal through a transmittal letter, ensuring compliance with the school's policies and ethical guidelines. Then, a consent form was provided to each student, informing them of the study’s purpose, process, data confidentiality, and voluntary participation. The total 90 participants were equally grouped into two groups:45 for the control and another 45 for the experimental. The classification was based on their first-quarter science grades, ensuring that both groups achieved homogeneity
Both groups received instruction on naming and writing the structures of functional groups, including alcohol, aldehyde, carboxylic acid, ester, ether, and ketone. A 120-item pre-test was initially administered to assess students’ prior knowledge. However, for analysis, only the test items specifically reflecting the topics taught later in the training were used to ensure alignment with the learning intervention.
To maintain instruction consistency and to preserve treatment fidelity, the teacher assigned to the experimental group underwent a training session conducted by the researcher. This session also included a detailed briefing on the aims, pedagogical pattern, suitable use of the improvised molecular kit, and how to implement the kit in the context. A teaching guide with scripted instructions and provided activities was also included. During the intervention, direct observations of the classrooms were conducted to ensure that the instructional procedures were being implemented as intended, specifically by observing instruction delivery using a fidelity checklist.
The control group was taught through the lecture method, while the experimental group received instruction using the lecture method integrated with an improvised molecular kit. Before implementation, students in the experimental group received training on how to handle the molecular kit to ensure its proper use.
The instruction and assessment for both groups were held face-to-face. At the end of the lessons, a post-test was administered to assess the concepts that had been learned. The whole instruction-assessment process lasted for 9 school days.Following the students’ performance, their perception of using an improvised molecular kit was collected through a 10-item questionnaire. Data analyses were conducted to evaluate the efficacy of the learning material and to interpret the gathered results.
Creation Improvised Molecular Kit
The images (Figure 1) shown are of raw materials, including recycled items such as empty roll-on deodorant containers, pen casings, and plastic caps. Grouped separately, the materials indicate a deliberate method of sorting and readying them for collection and preparation. The assortment of vibrant and clear plastic components is repurposed into a practical, creative product.

Figure 1. Raw Materials of the Improvised Molecular Kit
Materials, Tools, and Equipment
The materials and equipment used to make the Indigenous materials were the roll-on balls from different deodorant brands, the pump tube of different spray bottles, ink chambers of ball pens, scissors, and a soldering iron for making holes in each roll-on ball.
1. Roll-on ball-Represents atoms such as Carbon, Oxygen, Hydrogen, Chlorine, Bromine, and Nitrogen
2. Pump tubes-Represent two longer flexible double and triple bonds.
3. Ink chamber-Represents a single bond
Preparation of the Improvised Molecular Kit
1. Use the soldiering iron to make four holes with a diameter of 2.3 cm in the Carbon atoms in a tetrahedral structure of the roll-on ball to create four bonding sites around it.
2. Make two holes with a diameter of 1.1 cm from the two carbon atoms to attach the double-bond and triple-bond.
3. Make another two holes from the Hydrogen, Oxygen, Chlorine, Nitrogen, and Bromine atoms in a vertical dimension of 3.6 cm. For the oxygen, create two additional holes with a diameter of 1.1 cm for double and triple bondattachment.
4. Cut the ink chamber to a length of 4 cm.
5. Cut the pump tube to a length of 6 cm.
To Assemble the Molecular Kit:
1. Insert the ink chamber between the carbon atoms and gently push until it securely fits into the holes.
2. Insert the pump tubes if there is a double bond on the given molecular structure of functional groups.
3. Insert the pump tubes and ink chambers if there is a triple bond on the given molecular structure of functional groups.
4. Connect all the atoms, such as hydrogen and oxygen, using the pump tubes or ink chamber according to the molecular structure of the given functional group.
Features of the Improvised Molecular Kit
The molecular kit comprises ten carbon atoms, two hydrogen atoms, oxygen, bromine, chlorine, and nitrogen atoms. The bonds consist of 32 single bonds and six pieces each for double and triple bonds.
The molecular kit legend in Figure 2 provides a detailed breakdown of the components used to construct molecular models, including different atoms and bond representations. Atoms are color-coded, with black representing carbon, red representing hydrogen, green representing oxygen, yellow representing chlorine, blue representing bromine, and orange representing nitrogen, along with specified quantities for each. Bonds are represented using white ink chambers for single bonds and white pump tubes for double and triple bonds. Additionally, sample molecular structures of propanal and 2-pentanone illustrate the application of the kit in modeling organic compounds.
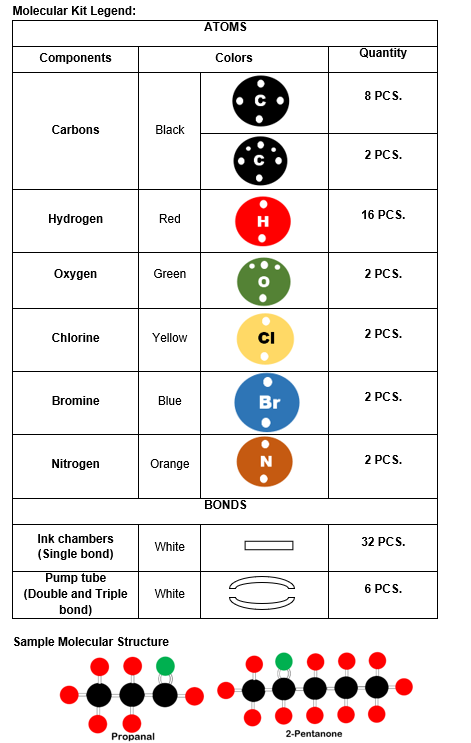
Figure 2. Molecular Kit Legend
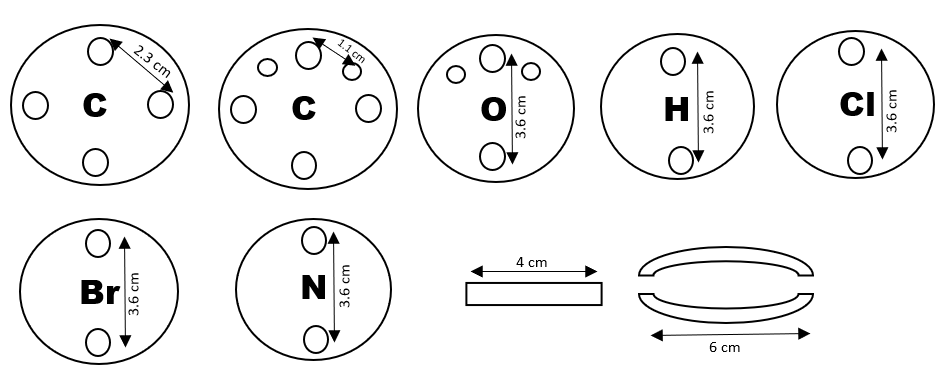
Figure 3. Atomic Models with Bonding Sites and Dimensions for Molecular Structure Assembly
The image (Figure 4) shows students assembling molecular structures using an improvised molecular kit. They are carefully connecting colored spheres and rods, which likely represent different atoms and bonds. The activity provides an engaging, hands-on learning experience to understand chemical structures, promoting student interaction and fostering real-world application of organic chemistry concepts.

Figure 4. Students Assembling the Molecular Structures
Data Analysis
Descriptive and inferential statistics were used to examine the students’ academic performance and their attitudes toward the use of an improvised molecular kit. For descriptive analysis, mean and standard deviation were computed to evaluate their scores and attitudes toward learning functional groups through alternative instructional materials. For inferential analysis, an ANCOVA was conducted to compare posttest scores between the experimental and control groups while controlling for pretest performance, thereby assessing the effectiveness of the improvised molecular kit. Prior to conducting ANCOVA, all necessary assumptions—including normality of distributions, homogeneity of variances, linearity, and homogeneity of regression slopes—were tested and found to be satisfied; thus, the use of ANCOVA was statistically appropriate. This combination of descriptive and assumption-verified inferential techniques ensured both an overview of the data and a valid, rigorous evaluation of the intervention’s impact.
Results
Pre-test and Post-test Performances of the Control and Experimental Groups
Table 1 presents the pretest and posttest results of respondents from control and experimental groups across six topics: Alcohol, Aldehyde, Carboxylic Acid, Ester, Ether, and Ketone. The pretest mean scores indicate that both groups initially "Did not meet the expectation" across all topics, suggesting a low baseline understanding. However, in the posttest, there was a notable improvement in scores for both groups. For the control group, posttest scores increased, with descriptions ranging from "Fairly Satisfactory" (5-8) to "Satisfactory" (9-12) across topics, demonstrating some improvement but still within a moderate level of understanding. In contrast, the experimental group exhibited a more significant increase in posttest scores, with descriptions ranging from "Satisfactory" (9-12) to "Very Satisfactory" (13-16). This suggests that the intervention applied to the experimental group enhanced their understanding of the topics more effectively than the control group. The findings indicate that while both groups improved their performance, the experimental group achieved higher post-test scores and better qualitative ratings, implying that the instructional strategy used for this group was more effective in facilitating learning.
Table 1. Respondents’ Pretest and Posttest
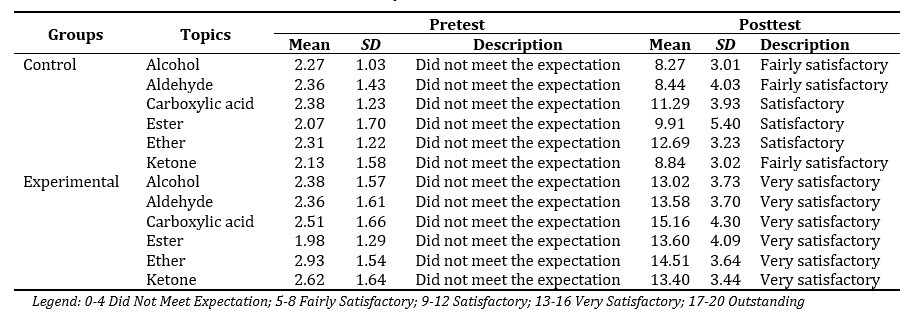
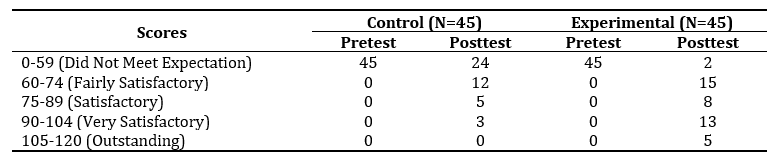
Table 2 presents a comparative analysis of the control and experimental group's performance in the pretest and posttest using the 120-item test. The pretest results show that all students in both control and experimental groups initially fell under the "Did Not Meet Expectation" category. After the intervention, the experimental group showed a significant improvement, with only 2 students remaining in the lowest category and 26 students reaching the “Satisfactory,” "Very Satisfactory," and "Outstanding" levels. In contrast,although the control group also improved, the gains were more modest, with the majority still falling within the lower performance categories.
Table 2. Comparative Analysis of Control and Experimental Group Performance in Pretest and Posttest
The assumption checks for ANCOVA indicated that all statistical requirements were satisfied, as shown in Table 3. The Shapiro–Wilk tests for both pretest and posttest scores in the control and experimental groups were not significant (p> .05), confirming the normality of score distributions. Levene’s test for homogeneity of variances yielded a non-significant result (F= 0.010,p= .919), indicating equal error variances across groups. A strong and significant positive relationship (r= 0.68,p< .001) between the covariate and dependent variable confirmed the linearity assumption. Finally, the non-significant interaction between pretest scores and group membership (F= 0.45,p= .505) showed that the regression slopes were homogeneous, validating the use of ANCOVA for the main analysis.
Table 3. Assumption Checks for ANCOVA
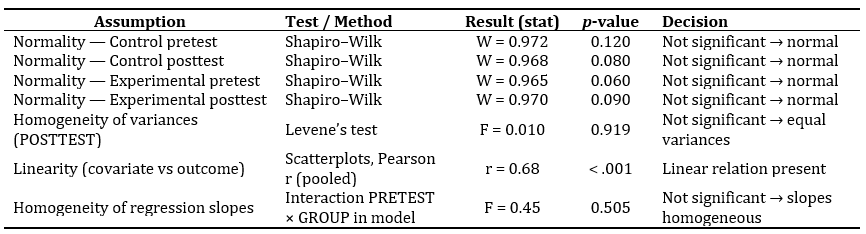
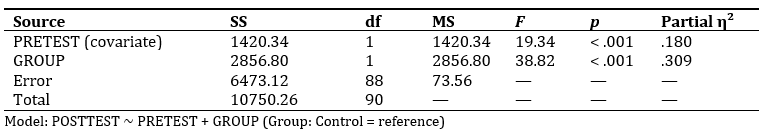
Table 4 presents the ANCOVA results, which revealed that after adjusting for pretest scores, there was a statistically significant effect of group on posttest performance, F(1, 88) = 38.82,p< .001, with a large effect size (Partial η² = .309). The covariate, pretest score, also had a significant influence on posttest results, F(1, 88) = 19.34, p < .001, indicating that baseline performance contributed meaningfully to the final scores. Adjusted means showed that the experimental group outperformed the control group, with mean posttest scores of 81.5 (95% CI: 76.0, 87.0) and 61.1 (95% CI: 55.6, 66.6),respectively. This substantial difference suggests that the use of the improvised molecular kit had a strong positive impact on students’ learning outcomes. Overall, the findings support the effectiveness of the intervention in enhancing academic performance beyond the influence of initial ability levels.
Table 4. ANCOVA Summary (adjusting posttest for pretest)
Attitude Towards the Use of the Improvised Molecular Kit
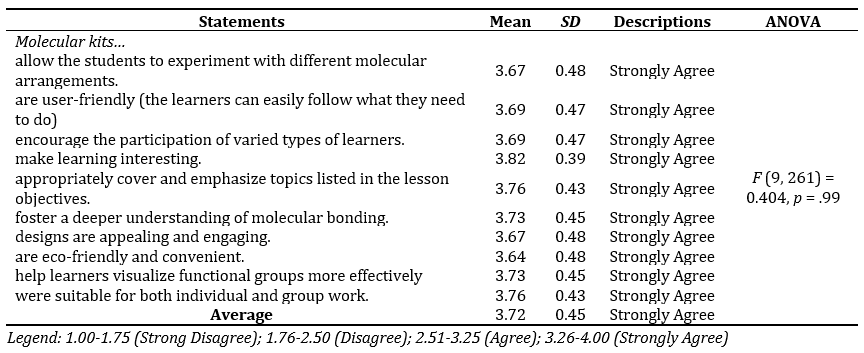
Table 5 shows that students strongly agree with the positive attributes of the improvised molecular kit, as reflected in the overall mean score of 3.72 (SD= 0.45). The highest-rated statement, "make learning interesting" (M = 3.82, SD= 0.39), suggests that the kit enhances student engagement. Additionally, students find the kit user-friendly (M = 3.69, SD= 0.47) and effective in encouraging participation among diverse learners. The kit supports lesson objectives (M = 3.76, SD= 0.43) and helps learners visualize molecular structures and functional groups (M = 3.73, SD= 0.45). The findings highlight the kit's effectiveness in fostering more profound understanding, engagement, and convenience in learning molecular concepts. The one-way repeated measures ANOVA revealed no statistically significant differences among the attitude items, F(9, 261) = 0.404, p= .99, indicating that students rated all items similarly and consistently.
Table 5. Attitude Towards the Use of the Improvised Molecular Kit
Discussion
The study's findings suggest that using improvised molecular kits in teaching across six topics —Alcohol, Aldehyde, Carboxylic Acid, Ester, Ether, and Ketone —can offer adaptable and powerful teaching resources, enhancing student performance and making experimental learning more widely available. The large effect size (Partial η² = .309) in ANCOVA also suggests that the intervention’s impact was not only statistically significant but also educationally meaningful.
Based on the constructivist standpoint, these kits provide learners with opportunities to build their own understanding of the chemical concepts of the six topics, which require active participation in investigating and manipulating the materials, rather than passively listening to the teacher. This hands-on interaction allows students to actively construct their own understanding by tapping into existing mechanisms in their brains. Through engaging with students in authentic contexts, the molecular kits support the development of conceptual understanding, the integration of personal experiences, and the formation of knowledge derived from students' interactions with the alternative materials. This experiential approach fosters deeper learning in students, rather than superficial rote learning, as they can test and refine their hypotheses, observe outcomes, and adjust their mental model, reflecting the fundamental principles of constructivist learning modes.
Additionally, the results provide an explanation for Cognitive Load Theory (CLT). Organic chemistry is highly abstract and has a high intrinsic cognitive load because of the difficulty in understanding how atoms are connected. Enabling improved experiential learning through improvised molecular kits provides actual, tangible, visual, and tactile context, supporting them and reducing the extraneous cognitive load associated with abstract concepts, thereby creating a more engaging experience. The use of these kits also allows for segmenting and scaffolding the learning process. Students can break down information bit by bit and facilitate chunked processing, thereby freeing up working memory for meaningful learning. When storing mental representations in physical models, they can be easily manipulated and laterally transformed to refine students' spatial and structural understanding, ultimately facilitating their long-term retention.
Furthermore, the results indicate that incorporating improvised materials and microscale kits into chemistry education can enhance student engagement, improve learning outcomes, and provide flexible and accessible alternatives to traditional laboratory experiences, particularly in resource-limited or remote learning environments. In constructivist learning theory, these resources encourage play, exploration, and engagement as students take responsibility for their own learning by experiencing and manipulating real, physical objects (Nagari et al., 2024). The effectiveness of improvised instructional materials enhances students' performance and retention in the chemistry classroom. For instance, Mushimiyimana et al. (2022) demonstrated that high school students taught using improvised materials outperformed those taught using traditional methods, significantly improving their chemistry performance. Similarly,Chukwunazo et al. (2022) found that secondary school students exhibited higher academic retention when taught with improvised instructional materials than standard ones. Additionally, Fechner and Sumfleth (2016) introduced microscale educational kits designed for at-home chemistry learning, emphasizing their safety, portability, and cost-effectiveness, which can be particularly beneficial in distance learning scenarios. Furthermore, Salta et al. (2022) reviewed hands-on laboratory experiments employing household supplies, highlighting their potential to foster students' laboratory competencies in remote settings.
The comparison of the mean gain of students’ concept retention indicates that the experimental group consistently achieved higher mean gains in concept retention across various organic chemistry topics than the control group. This suggests that the intervention effectively enhanced students' understanding and retention of these concepts. Similar findings have been reported in recent studies. For instance, Kaanklao and Suwathanpornkul (2020) applied Posner's approach, combined with design-based research, to develop a learning process that significantly improved students' achievement in organic chemistry and their conceptual comprehension. Additionally, Pokharna and Bharatiya (2021) found that promoting active learning increased interest in the subject. Furthermore, Grieger andLeontyev(2025) found that incorporating creative activities and exercises as assessment tools revealedstudents' conceptions in organic chemistry, aiding in the identification ofmisconceptions and enhancing learning outcomes. The studies mentioned underscore the effectiveness of innovative teaching strategies in improving concept retention in organic chemistry education.
Based on the perceived attitude towards using the improvised molecular kit, the students had a positive experience with the alternative resource, which significantly fostered hands-on learning and deepened conceptual understanding. Similarly, Stepek et al. (2020) developed a workshop in which high school students synthesized and assessed bioactive molecules to boost their engagement in teaching organic chemistry. Easa and Blonder (2024) also created customized pedagogical kits that addressed students' misconceptions, leading to improved self-efficacy and academic achievement. Furthermore, Veltri and Holland (2020) designed microfluidic devices to visualize the principles of stoichiometry, thereby enhancing students' understanding of challenging concepts. These findings imply that incorporating such kits into curricula can make chemistry more accessible and engaging, promoting scientific literacy and interest among students.
Conclusion
The results of the study suggest that students who use the improvised molecular kits exhibit better academic performance and more positive attitudes towards learning organic chemistry. Although gains in post-test scores were independent of experimental or control groups, the molecular hands-on group demonstrated significantly greater learning across all topics. Statistical analysis supported the effectiveness of the intervention, and students’ feedback was overwhelmingly favorable, with a strong emphasis on how the kit enhanced engagement, interaction, and understanding of the lessons.
However,to ensure these improvised molecular kits have a wider educational impact, considerations about scalability and sustainability are crucial. The use of the alternative intervention tool on a large-scale basis in various schools necessitated the development of cost-effective production schemes using locally available material or those that were recyclable. Similarly, schools would also require institutional support and access to programs that train teachers so they can more confidently work hands-on learning tools into their lessons. Without training or pedagogical guidance, the kits may not be employed to their maximum instructional potential.
Recommendations
Based on this conclusion, it is proposed that improvised molecular kits may be used in science teaching, particularly in organic chemistry. For science teachers, these kits offer a cost-effective solution to enhance student engagement and understanding of abstract concepts, such as molecular structure and functional groups. There should be extensive training for teachers on how to effectively leverage those tools in the classroom. In return, school administrators are being urged to support this work by allocating resources for the production or purchase of low-cost, locally sourced,or recycled materials used to assemble kits and provide access to professional development. For policymakers, there is a need to formalize the inclusion of molecular model kits as recommended instructional materials in science teaching for all schools, including those with under-resourced resources. Lastly, clear guidance and support systems—such as training modules and instructional resources—should be designed to foster nationwide, sustainable implementation of this hands-on, constructivist approach to chemistry education.
Limitations
A primary limitation of this study is its reliance on a quasi-experimental design with non-randomized groups, which may introduce selection bias and limit the generalizability of the results beyond the specific context of the study. Additionally, the research was conducted in a small public school with a relatively small sample size, and the improved kits' use was constructed from locally viable recycled materials, which may not be uniformly replicable in all settings. The short duration of the intervention also restricts the assessment of long-term retention and transfer of skills.
Ethics Statements
The studies involving human participants were reviewed and approved by the Graduate School and the Research and Development Office. The study conformed to Philippine Republic Act 10173, also known as the Data Privacy Act of 2012. Written informed consent was obtained from all participants, and for students under the age of 18, assent was secured alongside parental or guardian consent. To maintain anonymity, participants' names were not recorded or linked to any data, and responses were encoded using identification codes during analysis to ensure confidentiality.
The authors would like to thank the study participants and the experts for their contribution in completing this research endeavor. Also, we thank the Research and Development Office of Cebu Technological University–Danao Campus for their support and assistance
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This study received no financial support from any funding agency.
Generative AI Statement
As the authors of this work, we utilized the AI tool ChatGPT and Perplexity to enhance the construction of our research paper. After using this AI tool, we reviewed and verified the final version of our work. We, as the authors, take full responsibility for the content of our published work.
Authorship Contribution Statement
Cañete: Conceptualization, design, analysis, writing.Mutya: Editing/reviewing, supervision.

